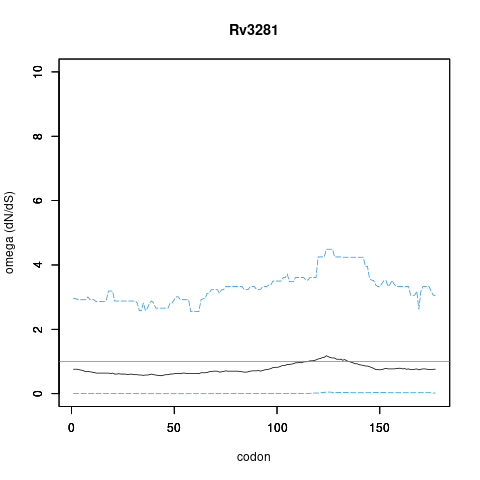
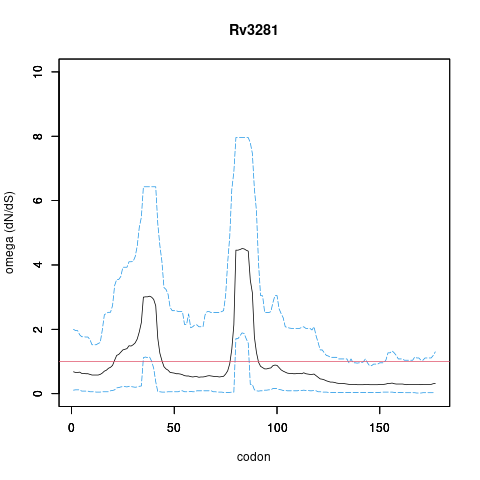
| Property | Value | Creator | Evidence | PMID | Comment |
| Citation | Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. G. Gago, D. Kurth et al. J. Bacteriol. 2006 | njamshidi | IDA | 16385038 | PMID: 16385038 AccE5 neede for max catalytic activity |
| Term | EC:6.4.1.2 Acetyl-CoA carboxylase. - IDA | njamshidi | IDA | 16385038 | PMID: 16385038 AccE5 neede for max catalytic activity
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Term | TBRXN:ACCOACr acetyl-CoA carboxylase, reversible reaction - IDA | njamshidi | IDA | 16385038 | PMID: 16385038 AccE5 neede for max catalytic activity
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Citation | Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. G. Gago, D. Kurth et al. J. Bacteriol. 2006 | njamshidi | ISS | 16385038 | PMID: 16385038 AccE5 neede for max catalytic activity |
| Term | EC:6.4.1.2 Acetyl-CoA carboxylase. - ISS | njamshidi | ISS | 16385038 | PMID: 16385038 AccE5 neede for max catalytic activity
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Term | TBRXN:ACCOACr acetyl-CoA carboxylase, reversible reaction - ISS | njamshidi | ISS | 16385038 | PMID: 16385038 AccE5 neede for max catalytic activity
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Citation | Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. G. Gago, D. Kurth et al. J. Bacteriol. 2006 | priti.priety | IDA | 16385038 | Spectrophotometric |
| Interaction | PhysicalInteraction Rv3280 | priti.priety | IDA | | Spectrophotometric
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Citation | Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. TW. Lin, MM. Melgar et al. Proc. Natl. Acad. Sci. U.S.A. 2006 | priti.priety | IDA | 16492739 | Spectrophotometric |
| Interaction | PhysicalInteraction Rv3280 | priti.priety | IDA | | Spectrophotometric
TW. Lin, MM. Melgar et al. Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 2006 |
| Citation | Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. G. Gago, D. Kurth et al. J. Bacteriol. 2006 | priti.priety | IDA | 16385038 | Structural Analysis |
| Interaction | PhysicalInteraction Rv3280 | priti.priety | IDA | | Structural Analysis
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Citation | Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. TW. Lin, MM. Melgar et al. Proc. Natl. Acad. Sci. U.S.A. 2006 | priti.priety | IDA | 16492739 | Structural Analysis |
| Interaction | PhysicalInteraction Rv3280 | priti.priety | IDA | | Structural Analysis
TW. Lin, MM. Melgar et al. Structure-based inhibitor design of AccD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 2006 |
| Name | Biotin-dependent propionyl-CoA carboxylase epsilon subunit involved in the synthesis of methyl-malonyl-CoA required for the biosynthesis of multiple methyl-branched fatty acids; the enzyme complex made of AccA3-AccD5-AccE5 shows a clear substrate preference for propionyl-CoA compared with acetyl-CoA (used in the generation of malonyl-CoA) | mjackson | IDA | | Claisen-type condensation |
| Other | TBPWY:Methyl-malonyl-CoA synthesis | mjackson | | | Biotin-dependent propionyl-CoA carboxylase epsilon subunit involved in the synthesis of methyl-malonyl-CoA required for the biosynthesis of multiple methyl-branched fatty acids (enzymatic); the enzyme complex made of AccA3-AccD5-AccE5 shows a clear substrate preference for propionyl-CoA compared with acetyl-CoA (used in the generation of malonyl-CoA)
TJ. Oh, J. Daniel et al. Identification and characterization of Rv3281 as a novel subunit of a biotin-dependent acyl-CoA Carboxylase in Mycobacterium tuberculosis H37Rv. J. Biol. Chem. 2006 |
| Citation | Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. G. Gago, D. Kurth et al. J. Bacteriol. 2006 | mjackson | | 16385038 | Biotin-dependent propionyl-CoA carboxylase epsilon subunit involved in the synthesis of methyl-malonyl-CoA required for the biosynthesis of multiple methyl-branched fatty acids (enzymatic); the enzyme complex made of AccA3-AccD5-AccE5 shows a clear substrate preference for propionyl-CoA compared with acetyl-CoA (used in the generation of malonyl-CoA) |
| Other | TBPWY:Methyl-malonyl-CoA synthesis | mjackson | | | Biotin-dependent propionyl-CoA carboxylase epsilon subunit involved in the synthesis of methyl-malonyl-CoA required for the biosynthesis of multiple methyl-branched fatty acids (enzymatic); the enzyme complex made of AccA3-AccD5-AccE5 shows a clear substrate preference for propionyl-CoA compared with acetyl-CoA (used in the generation of malonyl-CoA)
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Citation | Identification and characterization of Rv3281 as a novel subunit of a biotin-dependent acyl-CoA Carboxylase in Mycobacterium tuberculosis H37Rv. TJ. Oh, J. Daniel et al. J. Biol. Chem. 2006 | mjackson | | 16354663 | Biotin-dependent propionyl-CoA carboxylase epsilon subunit involved in the synthesis of methyl-malonyl-CoA required for the biosynthesis of multiple methyl-branched fatty acids (enzymatic); the enzyme complex made of AccA3-AccD5-AccE5 shows a clear substrate preference for propionyl-CoA compared with acetyl-CoA (used in the generation of malonyl-CoA) |
| Citation | Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. authors,KY. Rhee,LP. de Carvalho,R. Bryk,S. Ehrt,J. Marrero,SW. Park,D. Schnappinger,A. Venugopal,C. Nathan Trends Microbiol. 2011 | extern:JZUCKER | | 21561773 | Traceable author statement to experimental support |
| Term | EC:6.4.1.2 Acetyl-CoA carboxylase. - NR | extern:JZUCKER | NR | | Traceable author statement to experimental support
authors,KY. Rhee,LP. de Carvalho,R. Bryk,S. Ehrt,J. Marrero,SW. Park,D. Schnappinger,A. Venugopal,C. Nathan Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol. 2011 |
| Term | EC:6.4.1.3 Propionyl-CoA carboxylase. - NR | extern:JZUCKER | NR | | Traceable author statement to experimental support
authors,KY. Rhee,LP. de Carvalho,R. Bryk,S. Ehrt,J. Marrero,SW. Park,D. Schnappinger,A. Venugopal,C. Nathan Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol. 2011 |
| Citation | Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. G. Gago, D. Kurth et al. J. Bacteriol. 2006 | extern:JZUCKER | | 16385038 | Inferred from mutant phenotype |
| Term | EC:6.4.1.2 Acetyl-CoA carboxylase. - NR | extern:JZUCKER | NR | | Inferred from mutant phenotype
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |
| Term | EC:6.4.1.3 Propionyl-CoA carboxylase. - NR | extern:JZUCKER | NR | | Inferred from mutant phenotype
G. Gago, D. Kurth et al. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 2006 |