Rv2950c (fadD29)
Current annotations:
TBCAP: (community-based annotations - see table at bottom of page )
TBDB: long chain fatty acid CoA FadD26
REFSEQ: acyl-CoA synthetase
PATRIC: Long-chain fatty-acid-AMP ligase Mycobacterial subgroup FadD29
TUBERCULIST: Fatty-acid-AMP ligase FadD29 (fatty-acid-AMP synthetase) (fatty-acid-AMP synthase)
NCBI: Fatty-acid-AMP ligase FadD29 (fatty-acid-AMP synthetase) (fatty-acid-AMP synthase)
updated information (H37Rv4):
gene name: fadD29
function:
reference:
Coordinates in H37Rv: 3300596 - 3302455
Gene length: 1860 bp (with stop codon), 619 aa (without stop codon)
Operon:
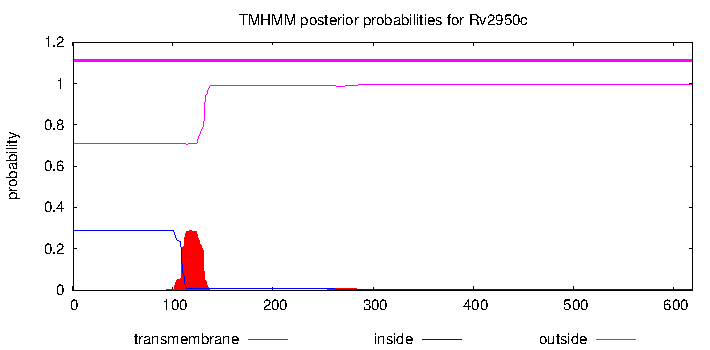
Trans-membrane region:
Role: I.A.3 - Fatty acids
GO terms:
Reaction(s) (based on iSM810 metabolic model):
Gene Expression Profile Gene Modules Orthologs among selected mycobacteria Protein structure:
Search for Homologs in PDB Top 10 Homologs in PDB (as of Nov 2020): PDB aa ident species PDB title 3E53 56% Mycobacterium tuberculosis Crystal structure of N-terminal domain of a Fatty Acyl AMP Ligase FAAL28 from Mycobacterium tuberculosis 3T5A 56% Mycobacterium tuberculosis Crystal structure of N-terminal domain of FAAL28 G330W mutant from Mycobacterium tuberculosis 5D6N 39% Mycobacterium smegmatis (strain ATCC 700084 / mc(2)155) Crystal structure of a mycobacterial protein 5ICR 38% Mycobacterium smegmatis (strain ATCC 700084 / mc(2)155) 2.25 Angstrom Resolution Crystal Structure of Fatty-Acid-CoA Ligase (FadD32) from Mycobacterium smegmatis in complex with Inhibitor 5'-O-[(11-phenoxyundecanoyl)sulfamoyl]adenosine. 5EY8 38% Mycobacterium smegmatis Structure of FadD32 from Mycobacterium smegmatis complexed to AMPC20 5D6J 38% Mycobacterium smegmatis (strain ATCC 700084 / mc(2)155) Crystal structure of a mycobacterial protein 5HM3 37% Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) 2.25 Angstrom Resolution Crystal Structure of Long-chain-fatty-acid-AMP Ligase FadD32 from Mycobacterium tuberculosis in complex with Inhibitor 5'-O-[(11-phenoxyundecanoyl)sulfamoyl]adenosine 5EY9 36% Mycobacterium marinum Structure of FadD32 from Mycobacterium marinum complexed to AMPC12
Links to additional information on fadD29:
Amino Acid Sequence
MKTNSSFHAAGEVATQPAWGTGEQAAQPLNGSTSRFAMSESSLADLLQKAASQYPNRAAYKFIDYDTDPAGFTETVTWWQVHRRAMIVAEELWIYASSGD
RVAILAPQGLEYIIAFMGVLQAGLIAVPLPVPQFGIHDERISSALRDSAPSIILTTSSVIDEVTTYAPHACAAQGQSAPIVVAVDALDLSSSRALDPTRF
ERPSTAYLQYTSGSTRAPAGVVLSHKNVITNCVQLMSDYIGDSEKVPSTPVSWLPFYHDMGLMLGIILPMINQDTAVLMSPMAFLQRPARWMQLLAKHRA
QISSAPNFGFELAVRRTSDDDMAGLDLGHVRTIVTGAERVNVATLRRFTERFAPFNLSETAIRPSYGLAEATVYVATAGPGRAPKSVCFDYQQLSVGQAK
RAENGSEGANLVSYGAPRASTVRIVDPETRMENPAGTVGEIWVQGDNVGLGYWRNPQQTEATFRARLVTPSPGTSEGPWLRTGDLGVIFEGELFITGRIK
ELLVVDGANHYPEDIEATIQEITGGRVVAIAVPDDRTEKLVTIIELMKRGRTDEEEKNRLRTVKREVASAISRSHRLRVADVVMVAPGSIPVTTSGKVRR
SASVERYLHHEFSRLDAMA
(
Nucleotide sequence available on
KEGG )
Additional Information
MtbTnDB - interactive tool for exploring a database of published TnSeq datasets for Mtb
TnSeqCorr
Rv2950c/fadD29,
gene len: 1859 bp, num TA sites: 47
condition dataset call medium method notes
in-vitro DeJesus 2017 mBio non-essential 7H9 HMM fully saturated, 14 TnSeq libraries combined
in-vitro Sassetti 2003 Mol Micro non-essential 7H9 TRASH essential if hybridization ratio<0.2
in-vivo (mice) Sassetti 2003 PNAS non-essential BL6 mice TRASH essential if hybridization ratio<0.4, min over 4 timepoints (1-8 weeks)
in-vitro (glycerol) Griffin 2011 PPath non-essential M9 minimal+glycerol Gumbel 2 replicates; Padj<0.05
in-vitro (cholesterol) Griffin 2011 PPath non-essential M9 minimal+cholesterol Gumbel 3 replicates; Padj<0.05
differentially essential in cholesterol Griffin 2011 PPath NO (LFC=0.06) cholesterol vs glycerol resampling-SR YES if Padj<0.05, else not significant; LFC<0 means less insertions/more essential in cholesterol
in-vitro Smith 2022 eLife non-essential 7H9 HMM 6 replicates (raw data in Subramaniam 2017, PMID 31752678)
in-vivo (mice) Smith 2022 eLife non-essential BL6 mice HMM 6 replicates (raw data in Subramaniam 2017, PMID 31752678)
differentially essential in mice Smith 2022 eLife NO (LFC=0.145) in-vivo vs in-vitro ZINB YES if Padj<0.05, else not significant; LFC<0 means less insertions/more essential in mice
in-vitro (minimal) Minato 2019 mSys non-essential minimal medium HMM
in-vitro (YM rich medium) Minato 2019 mSys non-essential YM rich medium HMM 7H9 supplemented with ~20 metabolites (amino acids, cofactors)
differentially essential in YM rich medium Minato 2019 mSys NO (LFC=0.5) YM rich vs minimal medium resampling
Analysis of Positive Selection in Clinical Isolates
*new*
data from Culviner et al (2025) (55,259 Mtb clinical isolates)
overall pN/pS for Rv2950c: 0.747349196
lineage-specific pN/pS in L1: 0.620185177
lineage-specific pN/pS in L2: 0.920485367
lineage-specific pN/pS in L3: 0.657684745
lineage-specific pN/pS in L4: 0.778360845
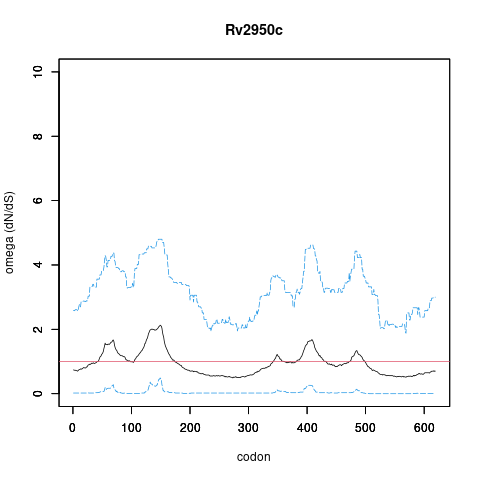
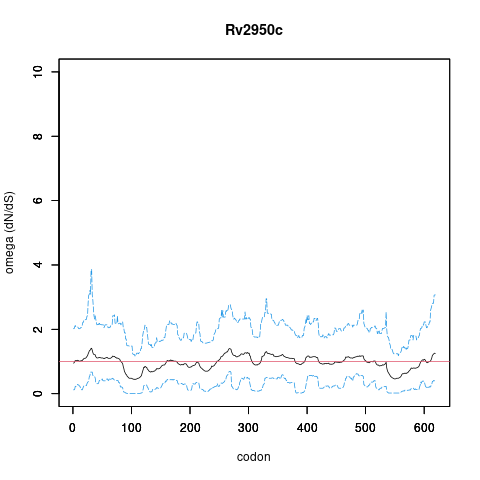
Analysis of dN/dS (omega) in two collections of Mtb clinical isolates using GenomegaMap (Window model) (see description of methods )
Moldova: 2,057 clinical isolates
global set: 5,195 clinical isolates from 15 other countries
In the omega plots, the black line shows the mean estimate of omega (dN/dS) at each codon, and the blue lines are the bounds for the 95% credible interval (95%CI, from MCMC sampling).
A gene is under significant positive selection if the lower-bound of the 95%CI of omega (lower blue line) exceeds 1.0 at any codon.
Moldova (2,057) global set (5,195)
under significant positive selection? NO NO
omega peak height (95%CI lower bound) 2.13 (0.49) 1.39 (0.69)
codons under selection
omega plots
genetic variants* link link
statistics at each codon link link
* example format for variants: "D27 (GAC): D27H (CAC,11)" means "Asp27 (native codon GAC) mutated to His (codon CAC) in 11 isolates"
TnSeq Data No data currently available.
No TnSeq data currently available for this Target.
RNASeq Data No data currently available.
No RNA-Seq data currently available for this Target.
Metabolomic Profiles No data currently available.
No Metabolomic data currently available for this Target.
Proteomic Data No data currently available.
No Proteomic data currently available for this Target.
Regulatory Relationships from Systems Biology
BioCyc
Gene interactions based on ChIPSeq and Transcription Factor Over-Expression (TFOE) (Systems Biology )
NOTE:
Green edges represent the connected genes being classified as differentially essential as a result of the middle gene being knocked out. These interactions are inferred based on RNASeq.
Interactions based on ChIPSeq data
RNA processing and modification
Energy production and conversion
Chromatin structure and dynamics
Amino acid transport and metabolism
Cell cycle control, cell division, chromosome partitioning
Carbohydrate transport and metabolism
Nucleotide transport and metabolism
Lipid transport and metabolism
Coenzyme transport and metabolism
Translation, ribosomal structure and biogenesis
Cell wall/membrane/envelope biogenesis
Replication, recombination and repair
Posttranslational modification, protein turnover, chaperones
Secondary metabolites biosynthesis, transport and catabolism
Inorganic ion transport and metabolism
General function prediction only
Intracellular trafficking, secretion, and vesicular transport
Signal transduction mechanisms
Differentially expressed as result of RNASeq in glycerol environment (Only top 20 genes shown sorted by log fold change with p_adj 0.05).
Conditionally essential as result of TNSeq (Only top 20 genes shown sorted by log fold change with p_adj 0.05).
Binds To:
No bindings to other targets were found.
Bound By:
No bindings from other targets were found.
Binds To:
No bindings to other targets were found.
Bound By:
Upregulates:
Does not upregulate other genes.
Upregulated by:
Not upregulated by other genes.
Downregulates:
Does not downregulate other genes.
Downregulated by:
Not downregulated by other genes.
Property Value Creator Evidence PMID Comment
Interaction Operon Rv2949c ashwinigbhat IDA Co-expression (Functional linkage)B. Abomoelak, EA. Hoye et al. mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. J. Bacteriol. 2009
Citation mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. B. Abomoelak, EA. Hoye et al. J. Bacteriol. 2009 ashwinigbhat IEP 19648248 Co-expression (Functional linkage)
Interaction Operon Rv2949c ashwinigbhat IEP Co-expression (Functional linkage)B. Abomoelak, EA. Hoye et al. mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. J. Bacteriol. 2009
Interaction Operon Rv2948c ashwinigbhat IEP Co-expression (Functional linkage)B. Abomoelak, EA. Hoye et al. mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. J. Bacteriol. 2009
Citation mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. B. Abomoelak, EA. Hoye et al. J. Bacteriol. 2009 ashwinigbhat IMP 19648248 Co-expression (Functional linkage)
Interaction Regulatory Rv0348 ashwinigbhat IMP Co-expression (Functional linkage)B. Abomoelak, EA. Hoye et al. mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. J. Bacteriol. 2009
Interaction RegulatedBy Rv0348 yamir.moreno IEP Microarrays. mRNA levels of regulated element measured and compared between wild-type and trans-element mutation (knockout, over expression etc.) performed by using microarray (or macroarray) experiments..B. Abomoelak, EA. Hoye et al. mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis. J. Bacteriol. 2009
Interaction RegulatedBy Rv1221 yamir.moreno IEP Microarrays. mRNA levels of regulated element measured and compared between wild-type and trans-element mutation (knockout, over expression etc.) performed by using microarray (or macroarray) experiments..R. Manganelli, MI. Voskuil et al. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol. Microbiol. 2001
Name Fatty acyl-AMP ligase involved in the biosynthesis of phenolic glycolipids; catalyzes the activation of hydroxyphenylalkanoates which are then transferred onto PpsA to yield phenolphthiocerol mjackson IMP Phthiocerol dimycocerosates (PDIM), phenolic glycolipids (PGL) and para-hydroxybenzoic acid derivatives
Name Fatty acyl-AMP ligase involved in the biosynthesis of phenolic glycolipids; catalyzes the activation of hydroxyphenylalkanoates which are then transferred onto PpsA to yield phenolphthiocerol mjackson IDA Phthiocerol dimycocerosates (PDIM), phenolic glycolipids (PGL) and para-hydroxybenzoic acid derivatives
Citation Delineation of the roles of FadD22, FadD26 and FadD29 in the biosynthesis of phthiocerol dimycocerosates and related compounds in Mycobacterium tuberculosis. authors,R. Simone,M. Lger,P. Constant,W. Malaga,H. Marrakchi,M. Daff,C. Guilhot,C. Chalut FEBS J. 2010 mjackson 20553505 Fatty acyl-AMP ligase involved in the biosynthesis of phenolic glycolipids; catalyzes the activation of hydroxyphenylalkanoates which are then transferred onto PpsA to yield phenolphthiocerol (phenotypic [mycobacterial recombinant strains]; enzymatic)
Other TBPWY:Phthiocerol dimycocerosates, PGL & pHBAD mjackson Fatty acyl-AMP ligase involved in the biosynthesis of phenolic glycolipids; catalyzes the activation of hydroxyphenylalkanoates which are then transferred onto PpsA to yield phenolphthiocerol (phenotypic [mycobacterial recombinant strains]; enzymatic)authors,R. Simone,M. Lger,P. Constant,W. Malaga,H. Marrakchi,M. Daff,C. Guilhot,C. Chalut Delineation of the roles of FadD22, FadD26 and FadD29 in the biosynthesis of phthiocerol dimycocerosates and related compounds in Mycobacterium tuberculosis. FEBS J. 2010